Answer:
 mmol of
mmol of
 is left after 20 s.
is left after 20 s.
Step-by-step explanation:
Initial concentration of
 =
=
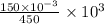 M = 0.333 M
M = 0.333 M
The integrated rate law for the given zero order reaction:
![[NH_(3)]=-kt+[NH_(3)]_(0)](https://img.qammunity.org/2021/formulas/chemistry/college/q50sylvccpz9torhpf0l0adoydn4gf05c7.png)
where,
![[NH_(3)]](https://img.qammunity.org/2021/formulas/chemistry/college/of5j2uziymt79fdj266v99mp6tp2ocp5o3.png) represents concentration of
represents concentration of
 after "t" time, k is rate constant and
after "t" time, k is rate constant and
![[NH_(3)]_(0)](https://img.qammunity.org/2021/formulas/chemistry/college/8flmlgms1cy7t8coojlm0sfrnf1n38gkzw.png) is initial concentration of
is initial concentration of
 .
.
Here, k = 0.0038 M/s,
![[NH_(3)]_(0)](https://img.qammunity.org/2021/formulas/chemistry/college/8flmlgms1cy7t8coojlm0sfrnf1n38gkzw.png) = 0.333 M and t = 20 s
= 0.333 M and t = 20 s
So,
![[NH_(3)]=(-0.0038M.s^(-1)* 20s)+0.333M](https://img.qammunity.org/2021/formulas/chemistry/college/ad6myga68mwm0rky0i2zrvp3aemb0ll82l.png)
or,
![[NH_(3)]](https://img.qammunity.org/2021/formulas/chemistry/college/of5j2uziymt79fdj266v99mp6tp2ocp5o3.png) = 0.257 M
= 0.257 M
So, number of mol of
 left after 20 s =
left after 20 s =
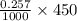 mol = 0.11565 mol
mol = 0.11565 mol
So, number of mmol of
 left after 20 s = 115.65 mmol =
left after 20 s = 115.65 mmol =
 mmol (2 sig. digits)
mmol (2 sig. digits)