Answer:
1×
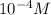
Explanation:
To solve this, you will need to do multiple steps.
Firstly, you will have to convert to pOH from pH, which is done by simply subtracting 14-10 to get a pOH of 4.
Then, use the following formula to calculate the [OH-} concentration:
[OH-]=
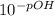
When converted to Scientific Notation, you get a resulting answer of
1×
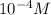 .
.