Complete question :
Consider the following piston-cylinder device whose piston is attached to a shaft and that contains 1.0 kg of air as its working fluid. The air can be modelled as an ideal gas with constant specific heats. The air has initial properties of P1 = 200 kPa and T1 = 300 K and is compressed in an adiabatic, reversible process until it reaches a maximum temperature of T2 = 800 K. Evaluate the specific heat capacities for air at a mean effective process temperature of 400 K.
(A) the initial specific volume, vi
(B) the final pressure, P2
(C) the final specific volume, V2
(D) the change in specific internal energy for the process, Aul-2
(E) the change in specific enthalpy, Ahi-2
(F) the change in specific entropy, Ast-2
(G) the total work done during the process, W.2
Answer:
a) 0.43 m³/kg
b) 6388.52 kPa
c) 0.0359 m³/kg
d) 363 kJ/kg
e) 506.5 kJ/kg
f) - 5.709*10¯⁴ kJ/kg
g) -362.905 kJ/kg
Step-by-step explanation:
Given:
P1 = 200kpa
T1 = 300K
T2 = 800K
m = 1.0 kg
Let's take at T = 400k
Cv = 0.726 kJ/kg
Cp = 1.013 kJ/kg
Air as fluid, R = 0.287kJ/kg
a)For the initial specific volume, let's use:
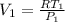
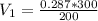
= 0.43 m³/kg
b) For final pressure, P2, let's use the formula :
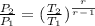
Solving for P2, we have:
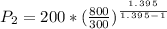
= 6388.52 kPa
c), To find specific volume, we use:
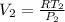
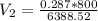
= 0.0359 m³/kg
d) Change in internal specific energy for the process:
Δ
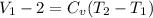
Δ
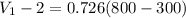
= 363 kJ/kg
e) Change in specific enthalpy:
Δ
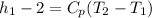
Δ
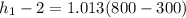
= 506.5 kJ/kg
f) For change in specific entropy, Let's use the formula:
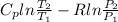
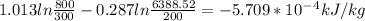
g) Total work done during the process.
Using the formula:
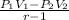
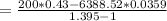
= - 362.905 kJ/kg