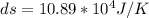Answer:
The change in entropy of the object is
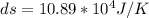
Step-by-step explanation:
From the question we are told that
The equation for the heat capacity is
![C = 14000(J)/(K) + (200 (J)/(K^2) )T + [3 (J)/(K^3) ] T^2](https://img.qammunity.org/2021/formulas/chemistry/college/fectyil95plc620z01ddblqyp4g4cvawd9.png)
The first temperature is
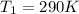
The first temperature is
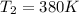
Generally the change in entropy is mathematically represented as
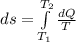
Where dQ is the change in the quantity of heat transferred with time which i mathematically represented as
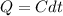
Substituting this above
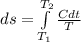
Substituting for C
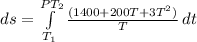
![ds = 1400\ ln [T]+ 200 (T^2)/(T) +3 (T^2)/(2 T) \ \ | \left \ T_2} \atop {T_1}} \right.](https://img.qammunity.org/2021/formulas/chemistry/college/9aq57dgb115qw7ehccapm7sgsodag6u62u.png)
![ds = 1400 ln [(T_2)/(T_1) ] + 200 (T_2 - T_1 ) + (3)/(2) (T_2^2 -T_1^2)](https://img.qammunity.org/2021/formulas/chemistry/college/yyqlziyyu0i4uo31u3kjfpm8p135ag976e.png)
Substituting values
![ds = 1400 ln [(380)/(290) ] + 200 (380 - 290 ) + (3)/(2) (380^2 -290 ^2)](https://img.qammunity.org/2021/formulas/chemistry/college/nbtu5xsabcgfstb2iw76gzkz67tnuxvky6.png)