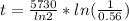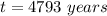Answer:
The wooden arrow is
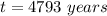 old
old
Step-by-step explanation:
From the question we are told that
The ratio of carbon -14 to carbon- 12 is
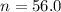 %
%
The half - life of carbon 14 is
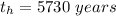
Generally half-life is mathematically evaluated as
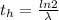
Where
 is the decay constant
is the decay constant
making
 the subject of the formula
the subject of the formula
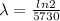
Now the age of the wooden arrow can be mathematically obtained
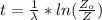
The initial amount of
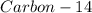 is
is
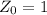
The amount
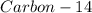 remaining in the wooden arrow is
remaining in the wooden arrow is
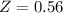 %
%
substituting values