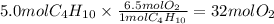Answer:
32 mol
Step-by-step explanation:
Step 1: Write the balanced equation.
C₄H₁₀ + 6.5 O₂ → 4 CO₂ + 5 H₂O
Step 2: Establish the appropriate molar ratio.
The molar ratio of C₄H₁₀ to O₂ is 1:6.5
Step 3: Calculate the moles of oxygen needed to burn 5.0 mol of butane.
We use the molar ratio established in the previous step.