Answer:
Its volume is 82.3 L
Step-by-step explanation:
The state of a quantity of gaseous matter is formulated based on four different variables: pressure, volume, temperature and number of moles of gas. Then, the equation known as the ideal gas equation, explains the relationship between these four variables using the expression:
P*V=n*R*T
where P represents the gas pressure, V its volume, n the number of moles of gas, R the gas constant and T the gas temperature.
In this case:
- R= 62.36365
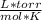
- T=28°C= 301°K (Being 0°C=273°K)
Replacing:
885 torr*V=3.88 mol*62.36365
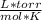 *301°K
*301°K
Solving:
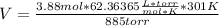
V=82.3 L
Its volume is 82.3 L