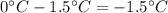Answer:
-1.5 °C
Step-by-step explanation:
The freezing-point depression is a colligative property, that is, the property of a solution. The freezing point of a solution is lower than the freezing point of the pure solvent. We can find the freezing-point depression of a solution of a non-volatile non-electrolyte solute using the following expression.
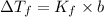
where,
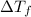 : the freezing-point depression
: the freezing-point depression
 : cryoscopic constant (For water, Kf = 1.86 °C/m)
: cryoscopic constant (For water, Kf = 1.86 °C/m)
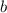 : molality
: molality

The freezing-point of pure water is 0°C. The freezing-point of the solution is: