Answer:
The answer to the question above is = 152.02 KJ/Kg
Step-by-step explanation:
Given:
Temperature at first state, (T
 )= 500k
)= 500k
Temperature at second state, (T
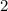 )= 500k
)= 500k
The above explains an isothermal process as a thermodynamic process,in which the temperature of the system remains constant
Pressure at first state, (p
 ) = 10 bar
) = 10 bar
Pressure at second state, (p
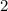 ) = 2 bar
) = 2 bar
The heat transfer=
Qrev/m= T x [s(T
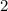 ) - s(T
) - s(T
 ) - R ㏑ (p
) - R ㏑ (p
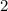 ÷ p
÷ p
 )]
)]
Isothermal means the temperature does not change, while Expansion means the volume has increased.
For the internal isothermal process:
Qrev/m= T x [- R ㏑ (p
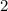 ÷ p
÷ p
 )]
)]
= 500 x - (8.314 ÷ 44.01) x In (2 ÷ 10) = 152.02 KJ/Kg
Energy equation at turbine is for the internally reversible isothermal process is:
Q-w = m [( (V
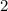 ²- V
²- V
 ²) ÷ 2) + g ( Z
²) ÷ 2) + g ( Z
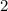 - Z
- Z
 )]
)]
where w= the most efficient work possible in J
Neglecting the effect of both potential and kinetic energy
(w/ m) = Qrev/ m
= 152.02 KJ/Kg