Answer:
The rate of effusion of helium is always about twice that of oxygen.
Step-by-step explanation:
Recall Graham's law of effusion:
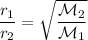
Where r are the rates of effusion and M are the respective molecular weights.
Let r₁ be the effusion rate of helium and r₂ be the effusion rate of oxygen. Hence, M₁ = 8.00 g/mol and M₂ = 32.00 g/mol.
Substitute:
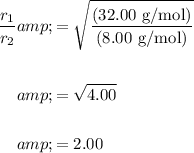
Solving for r₁ yields:
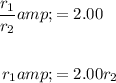
Hence, the rate of effusion of helium is always about twice that of oxygen. This is expected, as helium is smaller than oxygen.