Answer:
The volume will be 82.67 L
Step-by-step explanation:
Charles's Law is the relationship between the volume and temperature of a certain amount of ideal gas. In this way, Charles's law is a law that says that when the amount of gas and pressure are kept constant, the ratio between volume and temperature will always have the same value:
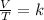
Having a certain volume of gas V1 that is at a temperature T1 at the beginning of the experiment, by varying the volume of gas to a new value V2, then the temperature will change to T2, and it will be true:
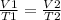
In this case, you know:
- V1= 40 L
- T1= 90 °C
- V2= ?
- T2= 186 °C
Replacing:
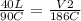
Solving:
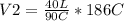
V2= 82.67 L
The volume will be 82.67 L