Answer:
The temperature would be 922.01 °K
Step-by-step explanation:
An ideal gas is characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T). The relationship between them constitutes the ideal gas law:
P*V=n*R*T
Where P is the gas pressure, V is the volume it occupies, T is its temperature, R is the ideal gas constant, and n is the number of moles of the gas.
In this case:
- P= 2300 mmHg
- V= 15 L
- n= 0.6 moles
- R= 62.36367
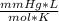
Replacing:
2300 mmHg* 15 L=0.6 moles*62.36367
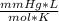 *T
*T
Solving:
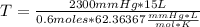
T= 922.01 °K
The temperature would be 922.01 °K