Answer:
12.5 g
Step-by-step explanation:
12.5 g of the compound would be formed.
First, let us look at the balanced equation of reaction.
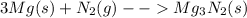
3 moles of Mg is required to react with 1 mole of N2 to produce 1 mole of product.
Recall that: mole = mass/molar mass
9.03 g of Mg = 9.03/24.3 = 0.3716 mole
3.48 g of N2 = 3.48/28 = 0.1243 mole
Mole ratio of Mg/N2 = 3:1
Hence, there is no limiting reactant.
3 moles of Mg is required for 1 mole of product.
0.3716 mole of Mg will therefore require:
0.3716 x 1/3 = 0.1239 moles of product.
Molar mass of product
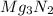 = 100.9 g/mol
= 100.9 g/mol
Mass of 0.1239 mole
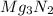 = mole x molar mass
= mole x molar mass
= 0.1239 x 100.9 = 12.5 g