Answer:
The mass of 0.280 mole sample of sodium hydroxide NaOH is 11.2 grams.
Step-by-step explanation:
To know the mass in grams of 0.280 moles of sample of sodium hydroxide NaOH, you must know the molar mass of the compound, that is, the mass of one mole of a substance, which can be an element or a compound.
So you know:
- Na: 23 g/mole
- O: 16 g/mole
- H: 1 g/mole
So, the molar mass of NaOH is:
NaOH= 23 g/mole + 16 g/mole+ 1 g/mole= 40 g/mole
Then the following rule of three can be applied: if in 1 mole of sodium hydroxide there are 40 grams, in 0.280 moles how much mass is there?
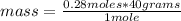
mass= 11.2 grams
The mass of 0.280 mole sample of sodium hydroxide NaOH is 11.2 grams.