Answer:
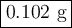
Step-by-step explanation:
We will need a balanced equation with masses, moles, and molar masses, so let’s gather all the information in one place.
Mᵣ: 74.55
2KClO₃ ⟶ 2KCl + 3O₂
V/mL: 50.0
1. Use the Ideal Gas Law to find the moles of O₂

2. Calculate the moles of KCl
The molar ratio is 2 mol KCl:1 mol O₂
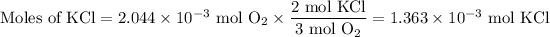
3. Calculate the mass of KCl
