Answer:
H₂O and H₂CO₃
Step-by-step explanation:
Per the Brønsted-Lowry theory, an acid is a substance that can donate a proton to another substance.
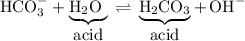
In the forward reaction, the water acts as a Brønsted-Lowry acid. It donates a proton to the bicarbonate ion and converts it to carbonic acid.
In the reverse reaction, the carbonic acid acts as a Brønsted-Lowry acid. It donates a proton to the hydroxide ion and converts it to water.