Question:
a. If Sr-90 has a half life of 28 years, how much Sr-90 remains?
b. How much total mass remains (Sr-90 and more stable isotopes combined)?
c. What quantity of more stable isotopes exist?
Answer:
a. 25/64 grams
b. 400 g
c. 399.61 g
Step-by-step explanation:
The formula for calculating half life is as follows;
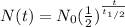
Where:
N(t) = Quantity of the remaining substance
N₀ = Initial radioactive substance quantity = 400
t = Time duration = 280 years
 = Half life of the radioactive substance = 28 years
= Half life of the radioactive substance = 28 years
a. Plugging in the values we have;
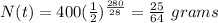
Therefore, the amount of Sr-90 that remains after 280 years is 25/64 or 0.391 grams
b. The amount of the total mass that remains is constant = 400 g
c. The quantity of the more stable isotopes that exits is therefore, 400 - 0.391 = 399.61 grams