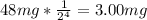Answer:
3.0 mg
Step-by-step explanation:
The half life of a substance is the time taken for the substance to reduce to half of its initial value. This reduction to half of the initial value is as a result of decay or emission of radiation. Half life can be used to calculate the age of organic objects using carbon dating.
Since the half life of tritium is 12.3 years, after 49.2 years the amount of half lives that have passed = 49.2 years / 12.3 years = 4 half lives passed.
The amount remaining if 48.0 mg is accidentally released from a nuclear power plant =
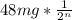 where n is the amount of half life = 4.
where n is the amount of half life = 4.
Therefore amount remaining =