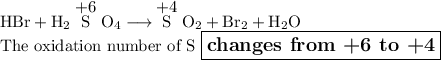Answer:
Yes
Step-by-step explanation:
The important rules for oxidation numbers are:
- The oxidation number of H in a compound is +1.
- The oxidation number of O in a compound is usually -2.
- The sum of the oxidation numbers in a compound is zero.
Thus, in your reaction, the oxidation numbers for S are: