Answer:
Approximately
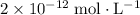 .
.
Step-by-step explanation:
The hydronium ion concentration
![\left[\mathrm{H_3O^(+)}\right]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/y1f04ul0bls3dm04wgyugpy51fr91uqt40.png) of an aqueous solution can be found from its
of an aqueous solution can be found from its
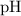 with the equation:
with the equation:
![\displaystyle \left[\mathrm{H_3O^(+)}\right] = 10^{-\mathrm{pH}}](https://img.qammunity.org/2021/formulas/chemistry/middle-school/niimd7udxlglgp8dcoyadqbs2279hhey0g.png) .
.
For this solution,
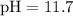 . Hence,
. Hence,
![\begin{aligned}& \left[\mathrm{H_3O^(+)}\right] \\ &= 10^{-\mathrm{pH}} \\ &= 10^(-11.7) \approx 2 * 10^(-12)\end{aligned}](https://img.qammunity.org/2021/formulas/chemistry/middle-school/smuscdx9xohzoo7jdtz514xhwjnu6tbkol.png) .
.
Note that for this equation, the number of significant figures in
![\left[\mathrm{H_3O^(+)}\right]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/y1f04ul0bls3dm04wgyugpy51fr91uqt40.png) should be the same as the number of decimal places in
should be the same as the number of decimal places in
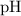 . For example, the
. For example, the
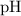 of this question comes with only one decimal place. As a result, there would be only one significant figure in the
of this question comes with only one decimal place. As a result, there would be only one significant figure in the
![\left[\mathrm{H_3O^(+)}\right]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/y1f04ul0bls3dm04wgyugpy51fr91uqt40.png) obtained from the equation.
obtained from the equation.