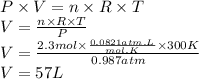Answer:
57 L
Step-by-step explanation:
Step 1: Convert the temperature to Kelvin
When working with gases, we always use the absolute temperature scale.
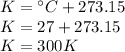
Step 2: Convert the pressure to atmospheres
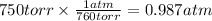
Step 3: Calculate the volume of the Ne sample
We will use the ideal gas equation.