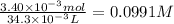Answer:
0.0991 M
Step-by-step explanation:
Step 1: Write the neutralization reaction between oxalic acid and sodium hydroxide.
H₂C₂O₄ + 2 NaOH = Na₂C₂O₄ + 2 H₂O
Step 2: Calculate the moles of oxalic acid
The molar mass of H₂C₂O₄ is 90.03 g/mol. The moles corresponding to 153 mg (0.153 g) are:
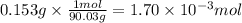
Step 3: Calculate the moles of sodium hydroxide
The molar ratio of H₂C₂O₄ to NaOH is 1:2.
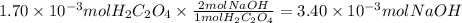
Step 4: Calculate the molarity of sodium hydroxide