Answer:
Per hour decay of the isotope is 0.96%.
Explanation:
Amount of radioactive element remaining after t hours is represented by
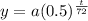
where a = initial amount
t = duration of decay (in hours)
Amount remaining after 1 hour will be,
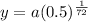
y = 0.9904a
So amount of decay in one hour = a - 0.9904a
= 0.0096a gms
Percentage decay every hour =
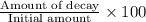
=
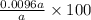
= 0.958 %
≈ 0.96 %
Therefore, per hour decay of the radioactive isotope is 0.96%.