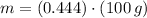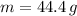Answer:
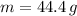
Step-by-step explanation:
The stoichometric formula for the electrolysis is:
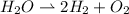
One mole of water is dissociated into 2 moles of hydrogen and one mole of oxygen. The mass ratio of hydrogen to water is:
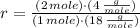
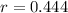
The amount of hydrogen that can be produced at 100 % efficiency is: