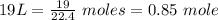Answer:
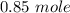
Step-by-step explanation:
Given: 19 L of oxygen gas
To find: Number of moles in 19 L of oxygen gas
Solution:
In International System of Units, the mole is the unit of measurement for amount of substance. A mole of a mole of particles is defined as exactly 6.02214076×10²³ particles. Particles may be atoms, molecules, ions, or electrons.
As 1 mole = 22.4 L, 1 L = 1/22.4 mole
So,