Answer:
79 extractions
Step-by-step explanation:
An isooctane/water partinioning constant of 12/1 means that 12 out of 13 parts of the Pb²⁺ complex will be found in isooctane, while the remaining 1 out of 13 part will remain in water.
- 12/13 * 100 = 92.3% of the Pb²⁺ will be removed with each extraction.
Now we convert 1.4 mg/mL to ug/mL:
- 1.4
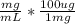 = 140 ug/mL
= 140 ug/mL
We're looking to have a final concentration of 0.25 ug/mL, so we can write:
- 140 mg/mL * (0.923)ⁿ = 0.25 ug/mL
Where n is the number of extractions.
We solve for n:
- n = ln (1.786x10⁻³) / ln (0.923)