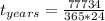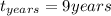Answer:
The time of the submersion is
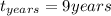
Step-by-step explanation:
From the question we are told that
The area of the plate is
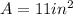
The mass of the corroded plate
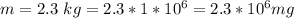
The corrosion penetration rate is
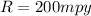
The density of steel is
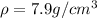
Generally the corrosion penetration rate can be mathematically represented as
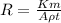
Where K is the corrosion imperial unit constant whose value is K = 534 mpy
t is the exposure time of the plate
Making t the subject of the formula
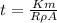
Substituting value
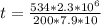
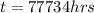
Converting to years