Answer:
Step-by-step explanation:
1. find the molar mass (amu) of each element and add them to get the whole molar mass.
2. divide the 1 element molar mass with the whole molar mass
3. multiple by 100 and that gives you the % composition.
56-57: NaCl
1. Na(22.99amu) + Cl (35.453amu)=58.443
2(Na):
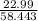 = .393
= .393
2(Cl):
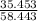 = .607
= .607
3(Na): .393 * 100=39.3%
3(Cl): .607 * 100= 60.7%
58-60
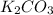
1. K: (39.098)(2)=78.196
_ C: (12.011)(1)= 12.011
_O: (15.99)(3) = 47.997
78.196+12.011+47.997= 138.204
2:K:
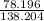 = .566 Step 3: (.566)(100)= 56.6%
= .566 Step 3: (.566)(100)= 56.6%
2: C:
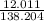 = .087 Step 3: (.087)(100)= 8.7%
= .087 Step 3: (.087)(100)= 8.7%
2: O:
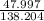 = .347 Step 3: (.347)(100) = 34.7%
= .347 Step 3: (.347)(100) = 34.7%
61-62
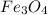
1. Fe (55.845)(3)= 167.535
_ O (15.999)(4) = 63.996
167.535+63.996=231.531
2: Fe:
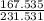 = .724 Step 3: (.724)(100)= 72.4%
= .724 Step 3: (.724)(100)= 72.4%
2: O :
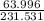 = .276 Step 3: (.276)(100) = 27.6%
= .276 Step 3: (.276)(100) = 27.6%
63-65
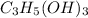
1.
C(12.011*3)=36.033
H(1.008*5)=5.04 + (1.008*3)=3.024 so its 8.064
O(15.999*3)=47.997
add them: 92.094
2: C:
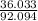 = .391 Step 3: (.391)(100) = 39.1%
= .391 Step 3: (.391)(100) = 39.1%
2: H:
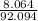 = .088 step 3: (.088)(100) = 8.8%
= .088 step 3: (.088)(100) = 8.8%
2: O:
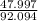 = .521 step 3: (.521)(100) = 52.1%
= .521 step 3: (.521)(100) = 52.1%