Answer:
1. 2NaN₃(s) → 2Na(s) + 3N₂(g)
2. 14.5 g NaN₃
Step-by-step explanation:
The answer is incomplete, as it is missing the required values to solve the problem. An internet search shows me these values for this question. Keep in mind that if your values are different your result will be different as well, but the solving methodology won't change.
" The airbags that protect people in car crashes are inflated by the extremely rapid decomposition of sodium azide, which produces large volumes of nitrogen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid sodium azide (NaN₃) into solid sodium and gaseous dinitrogen. 2. Suppose 71.0 L of dinitrogen gas are produced by this reaction, at a temperature of 16.0 °C and pressure of exactly 1 atm. Calculate the mass of sodium azide that must have reacted. Round your answer to 3 significant digits. "
1. The reaction that takes place is:
- 2NaN₃(s) → 2Na(s) + 3N₂(g)
2. We use PV=nRT to calculate the moles of N₂ that were produced.
P = 1 atm
V = 71.0 L
n = ?
T = 16.0 °C ⇒ 16.0 + 273.16 = 289.16 K
- 1 atm * 71.0 L = n * 0.082 atm·L·mol⁻¹·K⁻¹ * 289.16 K
Now we convert N₂ moles to NaN₃ moles:
- 0.334 mol N₂ *
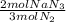 = 0.223 mol NaN₃
= 0.223 mol NaN₃
Finally we convert NaN₃ moles to grams, using its molar mass:
- 0.223 mol NaN₃ * 65 g/mol = 14.5 g NaN₃