Answer : The molarity of the resulting solution is, 0.29 M
Explanation :
When NaCl dissolved in water then it dissociates to give sodium ions and chloride ions.
Given,
Moles of NaCl = 0.87 mol
Volume of solution = 3.00 L
Molarity : It is defined as the number of moles of solute present in one liter of volume of solution.
Formula used :
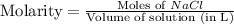
Now put all the given values in this formula, we get:
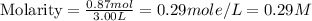
Therefore, the molarity of the resulting solution is, 0.29 M