Answer:
The time required for the coating is 105 s
Step-by-step explanation:
Zinc undergoes reduction reaction and absorbs two (2) electron ions.
The expression for the mass change at electrode
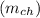 is given as :
is given as :
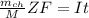
where;
M = molar mass
Z = ions charge at electrodes
F = Faraday's constant
I = current
A = area
t = time
also;
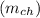 =
=
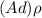 ; replacing that into above equation; we have:
; replacing that into above equation; we have:
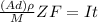 ---- equation (1)
---- equation (1)
where;
A = area
d = thickness
 = density
= density
From the above equation (1); The time required for coating can be calculated as;
![[ (20 cm^2 *0.0025 cm*7.13g/cm^3)/(65.38g/mol)*2 (moles\ of \ electrons)/(mole \ of \ Zn) * 9.65*10^4 (C)/(mole \ of \ electrons ) ] = (20 A) t](https://img.qammunity.org/2021/formulas/chemistry/college/z1dcfx2dy87zlb4m8xjdx9kcdbaf30ngmp.png)
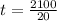
= 105 s