Answer: The molar mass of this gas is 221 g/mol
Step-by-step explanation:
The relation between density and molar mass is :
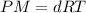
where P = pressure of gas = 1 atm (at STP)
M = molar mass of gas = 32 g/mol
d = density of gas = ?
R = gas constant =
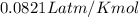
T= temperature of gas = 273 K ( at STP)
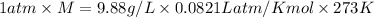
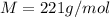
Thus the molar mass of this gas is 221 g/mol