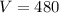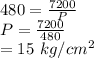Answer:
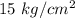
Explanation:
Given:
The volume of a gas "V" varies inversely with the pressure "P" put on it.
Volume is 360
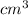 under a pressure of 20
under a pressure of 20
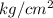
To find:
pressure when volume is 480
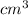
Solution:
As of a gas "V" varies inversely with the pressure "P" put on it,
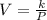
Here, k is a constant
As volume is 360
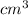 under a pressure of 20
under a pressure of 20
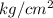 , put
, put
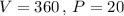
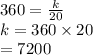
So,
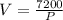
Put