Answer:
(A) 0.129 M
(B) 0.237 M
Step-by-step explanation:
(A) The reaction between potassium hydrogen phthalate and barium hydroxide is:
- 2HA + Ba(OH)₂ → BaA₂ + 2H₂O
Where A⁻ is the respective anion of the monoprotic acid (KC₈H₄O₄⁻).
We convert mass of phthalate to moles, using its molar mass:
- 0.978 g ÷ 156 g/mol = 9.27x10⁻³ mol = 9.27 mmol
Now we convert mmol of HA to mmol of Ba(OH)₂:
- 9.27 mmol HA *
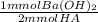 = 6.64 mmol Ba(OH)₂
= 6.64 mmol Ba(OH)₂
Finally we calculate the molarity of the Ba(OH)₂ solution:
- 6.64 mmol / 35.8 mL = 0.129 M
(B) The reaction between Ba(OH)₂ and HCl is:
- 2HCl + Ba(OH)₂ → BaCl₂ + 2H₂O
So the moles of HCl that reacted are:
- 17.1 mL * 0.129 M *
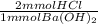 = 4.41 mmol HCl
= 4.41 mmol HCl
And the molarity of the HCl solution is:
- 4.41 mmol / 18.6 mL = 0.237 M