Answer:
Water of crystallization, X = 4.
Step-by-step explanation:
Molar mass of
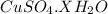
64 + 32 + (4x18) + x ( 1 × 2 + 16)
= 160 + 18x
Given: % water of crystallization (decrease in mass after heating) = 30%
⇒
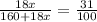
1800x = 31 (160 + 18x)
58.0645x = 160 + 18x
(58.0645 - 18)x = 160
x =
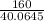 = 3.99 ≅ 4.
= 3.99 ≅ 4.
Water of crystallization, X = 4.