Answer : The mass of magnesium is, 2.99 grams.
Explanation :
As we know that 1 mole of magnesium contains
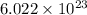 atoms
atoms
And, 1 mole of magnesium has 24.30 g
So, we can say that:
As,
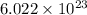 atoms of magnesium present in 24.30 gram of magnesium
atoms of magnesium present in 24.30 gram of magnesium
So,
 atoms of magnesium present in
atoms of magnesium present in
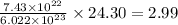 gram of magnesium
gram of magnesium
Therefore, the mass of magnesium is, 2.99 grams.