Answer: The concentrations of
 at equilibrium is 0.023 M
at equilibrium is 0.023 M
Step-by-step explanation:
Moles of
 =
=
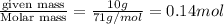
Volume of solution = 1 L
Initial concentration of
 =
=
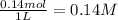
The given balanced equilibrium reaction is,
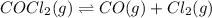
Initial conc. 0.14 M 0 M 0M
At eqm. conc. (0.14-x) M (x) M (x) M
The expression for equilibrium constant for this reaction will be,
![K_c=([CO]* [Cl_2])/([COCl_2])](https://img.qammunity.org/2021/formulas/chemistry/college/3xerdyepbs6wcdoen6yw52icx3gqax6e1l.png)
Now put all the given values in this expression, we get :
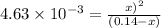
By solving the term 'x', we get :
x = 0.023 M
Thus, the concentrations of
 at equilibrium is 0.023 M
at equilibrium is 0.023 M