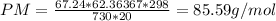Answer:
The molar mass is 85.59 g/mol
Step-by-step explanation:
Given:
m = mass = 67.24 g
V = Volume = 20 L
P = pressure = 730 mmHg
Question: What is the molar mass of the gas, PM = ?
The molecular weight of any susbtance is defined as the amount of mass over the number of moles.
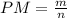
According to the ideal gas law:

Clearing the number of moles from the first equation and replacing it in the second
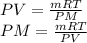
Here,
R = constant = 62.36367 mmHg L/mol K
T = 25°C = 298 K, we assume that the temperature is the standard.
Substituting values: