Answer:
The heat will begin to flow from the room temperature water into the ice.
Step-by-step explanation:
Hi there,
To get started, recall the principles of heat flow, and the laws of thermodynamics. Most importantly, remember that energy in the form of heat flows from a mass with higher heat towards a mass with lower heat.
In this case, ice has less heat than the room temperature water. Thus, when placed in the beaker, the heat will begin to flow from the room temperature water into the ice, warming up the ice, and melting it into water. The system will then reach a single final temperature, where it will be in equilibrium.
Final temperature of a solution with just two water masses (like ice and room temp water) can be calculated with the following formula:
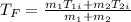 where m is mass, and Ti is initial temperature. Though this is not needed for this problem.
where m is mass, and Ti is initial temperature. Though this is not needed for this problem.
If you liked this solution, hit Thanks or give a Rating!
thanks,