Answer: The Molarity of the sulphate ion in a 2M
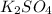 solution is 2M
solution is 2M
Step-by-step explanation:
Molarity is defined as the number of moles of solute dissolved per liter of the solution.
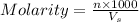
where,
n= moles of solute
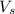 = volume of solution in ml
= volume of solution in ml
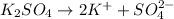
Now as 1 mole of
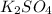 gives = 1 mole of
gives = 1 mole of
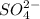
2 moles of
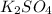 gives =
gives =
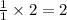 moles of
moles of
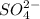
Thus Molarity of the sulphate ion in a 2M
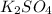 solution is 2 M
solution is 2 M