Answer:
The concentration of the solution is 2.86 M
Step-by-step explanation:
Molarity is a unit of concentration based on the volume of a solution. It is defined as the number of moles of solute that are dissolved in a given volume. In other words, molarity is defined as the number of moles of solute per liter of solution.
The Molarity of a solution is determined by the following expression:
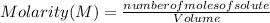
Molarity is expressed in units (
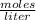 ).
).
In this case, you must then know the number of moles of HF, for which you must know the molar mass. Being:
the molar mass of HF is: HF= 1 g/mole + 19 g/mole= 20 g/mole
Then the following rule of three applies: if 20 g of HF are available in 1 mole, 14.3 g in how many moles will they be?
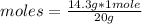
moles= 0.715
So:
- number of moles of solute: 0.715 moles
- Volume: 250 mL=0.250 L (being 1 L=1000 mL)
Replacing:
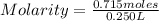
Solving:
Molarity= 2.86
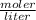 =2.86 M
=2.86 M
The concentration of the solution is 2.86 M