Answer : The moles of water present in solution are, 0.8297 moles.
Explanation :
First we have to calculate the mass of water.
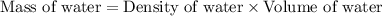
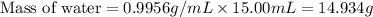
Now we have to calculate the moles of water.
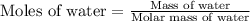
Molar mass of water = (2 × Molecular weight of hydrogen) + Molecular weight of oxygen
Molar mass of water = (2 × 1.00g/mol) + 16.00 g/mol
Molar mass of water = 18.00 g/mol
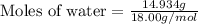
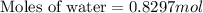
Therefore, the moles of water present in solution are, 0.8297 moles.