Answer:
Here's what I get
Step-by-step explanation:
Assume you have 0. 1 mol·kg⁻¹ solutions of A) KCl, B) CH₃OH, C) Ba(OH)₂, and D) CH₃COOH.
The formula for freezing point depression is
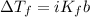
Where
b = the molal concentration
K_f = the freezing point depression constant
i = the van't Hoff i factor
The i-factor is the number of solute particles produced by one formula unit of the substance.
The only difference in the solutions is the i-factor of the solutes.
A) KCl
KCl(aq) ⟶ K⁺(aq) +Cl⁻(aq); i = 2
B) CH₃OH
Methanol is a nonelectrolyte.
CH₃OH(aq) ⟶ CH₃OH(aq); i = 1
C) Ba(OH)₂
Ba(OH)₂(aq) ⟶ Ba²⁺(aq) + 2OH⁻(aq); i = 3
D) CH₃COOH
CH₃COOH(aq) ⇌ CH₃COO⁻(aq)+ H⁺(aq); i ⪆ 1
The Ba(OH)₂ solution has the greatest i-value. It therefore has the greatest freezing point depression and the lowest freezing point.