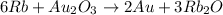Answer : The balanced chemical reaction will be:
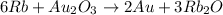
Explanation :
As per question, when rubidium react with gold oxide then it gives rubidium oxide and gold.
This reaction is a single replacement reaction in which more reactive element displaces the least react element from its solution.
The balanced chemical reaction will be: