Answer:
If 16 grams of sugar are in a spoonful, 2.83*10²² molecules of sugar are in the spoonful
Step-by-step explanation:
Avogadro's Number or Avogadro's Constant is the number of particles that make up a substance (usually atoms or molecules) and that can be found in the amount of one mole of that substance. Its value is 6.023 * 10²³ particles per mole. The Avogadro number applies to any substance.
So you need to know how many moles are 16 grams of sugar, C₁₂H₂₂O₁₁. For that you must know the molar mass of sugar. Being:
- C: 12 g/mole
- H: 1 g/mole
- O: 16 g/mole
the molar mass of sugar is:
C₁₂H₂₂O₁₁= 12 *12 g/mole + 22 * 1 g/mole + 11* 16 g/mole= 342 g/mole
Then the following rule of three applies: if 342 grams of sugar are present in 1 mole of substance, 16 grams in how many moles will they be?
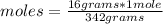
moles= 0.047
Now, knowing Avogadro's number, you can apply the following rule of three: if 1 mole of sugar contains 6.023 * 10²³ molecules, 0.047 moles how many molecules does it have?
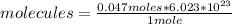
molecules= 2.83*10²²
If 16 grams of sugar are in a spoonful, 2.83*10²² molecules of sugar are in the spoonful