Answer:
At 468.57 atm pressure the gas volume would change to 28 L.
Step-by-step explanation:
Boyle's law states that the pressure of a gas in a closed container is inversely proportional to the volume of the container, when the temperature is constant.
This law can be expressed mathematically as:
P · V = k
where P is pressure, V is volume and k is a constant.
This formula can be used to determine the change in pressure or volume during an isothermal transformation (i.e. the temperature is constant) as follows:
P1 · V1 = P2 · V2
In this case,
- P1= 410 atm
- V1= 32 L
- P2= ?
- V2= 28 L
Replacing:
410 atm*32L= P2*28 L
Solving:
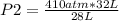
P2=468.57 atm
At 468.57 atm pressure the gas volume would change to 28 L.