Answer:
Gold.
Step-by-step explanation:
The specific heat is the measure to determine which material needs a lesser amount of energy to rise its temperature. Specific heats are described below:
Aluminium:
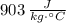
Gold :
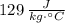
Water:
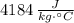
Wood:
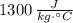
Gold reports the lowest specific heat and, hence, requires the least energy to raise its temperature 7°C.