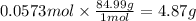Answer:
4.87 g
Step-by-step explanation:
Step 1: Calculate the moles of sodium nitrate
Considering the Avogadro's number, in 1 mole of sodium nitrate there are 6.02 × 10²³ formula units of sodium nitrate. We will use this ratio to find the moles corresponding to 3.45 × 10²² formula units.
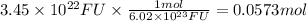
Step 2: Calculate the mass corresponding to 0.0573 moles of sodium nitrate
The molar mass of sodium nitrate is 84.99 g/mol. The mass corresponding to 0.0573 moles is: