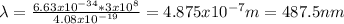Answer:
the wavelength, in nm, of the photon is 487.5 nm
Step-by-step explanation:
Given:
n = 4 (excited)
n = 2 (relaxes)
Question: Calculate the wavelength, in nm, λ = ?
First, it is important to calculate the energy of the electron when it excited and then when it relaxes.
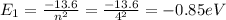 (excited)
(excited)
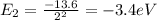 (relaxes)
(relaxes)
The change of energy
ΔE = E₁ - E₂=-0.85 - (-3.4) = 2.55 eV = 4.08x10⁻¹⁹J
For a photon, the wavelength emitted
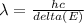
Here
h = Planck's constant = 6.63x10⁻³⁴J s
c = speed of light = 3x10⁸m/s
Substituting values: