Answer:
The longest wavelength is
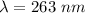
It is not in the range of visible light
Step-by-step explanation:
From the question the question we are told that
The binding energy is
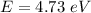
the kinetic energy of the ejected photoelectron is mathematically represented as
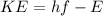
Where h is the plank constant with a values of
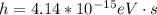
f is the frequency of the the EM which is mathematically represented as
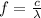
Here c is the speed of light with value

 is the wavelength
is the wavelength
So we have
![KE = h[(c)/(\lambda) ] - E](https://img.qammunity.org/2021/formulas/physics/college/l5ls4fk7cyak9jwdm4tplrf1ldf1qzc4ye.png)
So making
 the subject of the formula
the subject of the formula
![\lambda = (hc)/([KE +E ])](https://img.qammunity.org/2021/formulas/physics/college/1ioesub4i4ti9ey1ith6adb2v9jdqhfyxu.png)
Here the maximum kinetic energy is zero this is because out of all the electron ejected using a light of a threshold frequency (i.e a photo electron ) the one that has the maximum kinetic energy is none so this implies that maximum kinetic energy is zero so the equation becomes
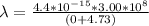
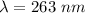
Looking at this we see that it is not in the range of visible light which is
400nm - 700nm